| Standard | |
| Limiting Reactant |
![]()
Stoichiometry consists of the calculations in chemistry that involve how much of each reactant is required to make the products of the reaction. The coefficients in the balanced equation are used to determine the number of moles of each element that are required in the reaction. Therefore, from the equation,
3H2 + N2 --> 2NH3
you can surmise that to make 2 moles of ammonia you need three moles of hydrogen gas and one mole of nitrogen gas. Yes, I know that almost everyone reading this page is saying that hey that sounds really cool, but what good is it to know how many moles you need to make something. I have the answer, if you know how many moles you need you can convert that to grams. On the periodic table there is an atomic weight. The atomic weight is equal to the number of grams in one mole of the element. If you have more than one element in a compound add the number of grams together according to the subscripts.
Examples:
How many moles of O2 are produced when 7.5 moles of KClO3 decompose according to the following equation?
KClO3 --> KCl + O2
Balance the Equation
2KClO3 --> 2KCl + 3O2
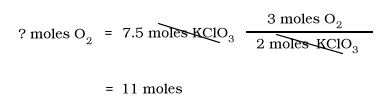
Al + HCl --> AlCl3 + H2
Balance the Equation
2Al + 6HCl --> 2AlCl3 + 3H2
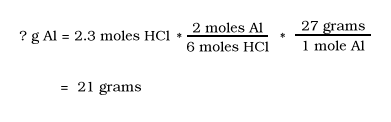
N2 + H2 --> NH3
Balance the Equation
N2 + 3H2 --> 2NH3

![]()
![]()
Now you say that stoichiometry is great, but what if you don't have such pretty numbers that work out evenly? That is where limiting reactant comes into play. In most situations one reactant will run out before the other. The reactant that is totally consumed is called the limiting reactant because it stops the reaction. Any other reactant that does not run out is called the excess reactant. Therefore the amount of product that can be produced is directly related to the limiting reactant.
The first step in a limiting reactant question is to determine the limiting reactant. To do this, for all reactants calculate the amount of product that would be produced if all of the reactant was used and there was excess of all the other reactants. The reactant that has the least product is the limiting reactant.
The amount of products is due to the amount of the limiting reactant.
An example:
Using the following reaction
Zn(s) + 2HCl(aq) --> ZnCl2(aq) + H2(g)
Which is the limiting reactant and how much H2 is produced if there is 0.30 mol of Zn and 0.52 moles of HCl?
Step one:
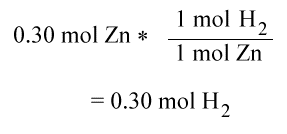
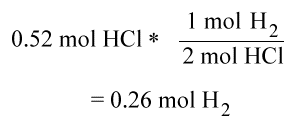
Since HCl is the limiting reactant, the number of moles of H2 produced is .26 moles.
![]()
![]()