Typically, we recognize acids and bases by their simple properties, such as taste. We know that a lemon is sour, so it is acidic. Bases tend to taste bitter. Acids and bases also change the color of certain dyes, such as phenolphthalein and litmus. Acids change litmus treated paper from blue to red. Acids change basic phenolpthalein from red to colorless. Bases change litmus treated paper from from red to blue and phenolphthalein from colorless to pink. Acids and bases neutralize the action of each other. This is why we take antacids for stomachaches, because the antacid is a base, and neutralizes the acid in the stomach.
| Arrhenius Concept of Acids and Bases | |
| Bronsted-Lowery Concept of Acids and Bases | |
| Lewis Concept of Acids and Bases | |
| Equilibrium with Acids and Bases |
![]()
This guy named Arrhenius concocted the first successful concept of
acids and bases. He did this by defining acids and bases according to the
effect these substances have on water. The Arrhenius concept of acids and
bases
is as follows: an acid is a
substance that when dissolved in water increases the concentration of the
hydrogen ion, H+. A base is a substance that when dissolved
in water increases the concentration of the hydroxide ion, OH-.
The hydrogen ion, is not just a bare proton, it is a proton bonded to a water molecule, H2O. This results in a hydronium ion, H3O+.
In Arrhenius's thoery, something that is a strong acid is a substance that completely ionizes in aqueous solution to give a hydronium ion, H3O+, and an anion. An anion is a negatively charged ion. An example of a strong acid is perchloric acid:
What is going on above is that we have perchloric acid in an aqueous soluion. This perchloric acid ionizes entirely and results in an hydronium ion and a perchlorate anion. Some other examples of strong acids would be: HI, HBr, HCl, HNO3, and H2SO4.
Now on to bases...A strong base is something that completely ionizes in aqueous solution to give a hydroxide ion and a cation. A cation is a positively charged ion. Strong bases are most of the hydroxides of Group IA elements and Group IIA elements including LiOH, NaOH, KOH, Ca(OH)2, Sr(OH)2 and Ba(OH)2.
Many of the acids and bases that we encounter in our everyday lives are not strong acids, they are considered weak. Weak acids do not completely ionize in solution, but exist in equilibrium. Let's look at the reaction for acetic acid:
![]()
![]()
The Bronsted-Lowery concept of acids and bases is that acid-base reactions can be seen as proton-transfer reactions. This results in acids and bases being able to be defined in terms of this proton (H+) transfer. According to the Bronsted-Lowery concept, acids donate a proton in a proton-transfer reactions. Bases accept the proton in a proton-transfer equation. As an example, lets look at the reaction of hydrochloric acid with ammonia. When we write it as an ionic equation we get:
What happens in this reaction in aqueous solution is a proton transfer. According to the Bronsted-Lowery concept, acids donate a proton in a proton-transfer reactions. Bases accept the proton in a proton-transfer equation. As an example, lets look at the reaction of hydrochloric acid with ammonia shown above. What happens in this reaction in aquesous solution is that a poton is transferred from H3O+ to NH3. This results in H3O+ losing a (H+), resulting in H2O. The NH3 gains the transferred proton, resulting in NH4+. We call H3O+ the proton donor, or acid. We call NH3 the proton acceptor, or base.
In the Bronsted-Lowery concept we have found that:
1. A base is a species that accepts protons, while an acid is a species that dontates protons.
2. Acids and bases can be ions as well as molecular substances.
3. Some species can act as either acids or bases, depending on what the other reactant is.
![]()
![]()
The Lewis concept of acids is generalized to include reactions of
acidic
and basic oxides and many other reactions. A Lewis acid is something that
can form a covalent bond by accepting an electron air from another
species. A Lewis base is something that can form a covalent bond by
donating an electron pair to something else. The Lewis and
Bronsted-Lowery
concepts are different ways of looking at the same chemical reactions.
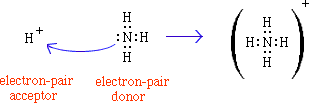
Here is a reaction in which an electron pair is transferred. The proton
(H+) is electron pair acceptor, alewis acid. NH3
has a lone pair of electrons and is a Lewis Base.
Whether or not an aqueous solution is neutral, acidic or basic
depends on the hydrogen-ion concentration. We give the acidity of an
aqueous solution in terms of the pH. pH is defined as the negative
logarithm of the molar hydrogen-ion concentration. A pH of 7 means that a
solution is neutral. A pH of below 7 means that a solution is acidic; a pH
of above 7 means that a solution is basic.
For example, let's say that we have a glass of frosty orange juice. This orange juice has a hydrogen-ion concentration of 2.9 x 10-4 M. What is the orange juice's pH?
The pH of this solution is less than 7 so this orange juice is acidic.
We can also find pH by solving for the hydroxide-ion concentration of a
solution. The measure of the hydroxide-ion concentration is called pOH.
Since we know that the pH scale goes from 0 to 14, we find that:
pH + pOH = 14
Let's say that we want to find the pH of an ammonia solution that has a hydroxide-ion concentration of 1.9 x 10-3 M. We start by finding the pOH.
Now we want to find the pH by subtracting:
![]()
![]()
Have you read the section about equilibrium yet? If you haven't this most likely won't make any sense to you. If you have, lets join in on the fun of acid-base equilibrium.
Remember Kc from the equilibrium section? It's back, and more useful than ever. Now to distinguish between the Kc of acids and bases we use Ka and Kb. (a for acids and b for bases) The equilibrium that is calculated in acids is usually the disaccociation of the H+ ions and the rest of the molecule. The weak acids and bases are the only ones that have Ka's and Kb's because in the strong acids dissociation is very close to 100%.
| Substance | Formula | Ka |
|---|---|---|
| Acetic Acid | HC2H3O2 | 1.7 x 10-5 |
| Benzoic Acid | HC7H5O2 | 6.3 x 10-5 |
| Boric Acid | H3BO3 | 5.9 x 10-10 |
| Carbonic Acid | H2CO3 | 4.3 x 10-7 |
| HCO3- | 4.8 x 10-11 | |
| Cyanic Acid | HCNO | 3.5 x 10-4 |
| Formic Acid | HCNO2 | 1.7 x 10-4 |
| Hydrocyanic Acid | HCN | 4.9 x 10-10 |
| Hydrofluric Acid | HF | 6.8 x 10 |
| Hydrogen Sulfate ion | HSO4- | 1.1 x 10-2 |
| Hydrogen Sulfide | H2S | 8.9 x 10-8 |
| HS- | 1.2 x 10-13 | |
| Hypochlorous acid | HClO | 3.5 x 10-8 |
| Nitrous Acid | HNO2 | 4.5 x 10-4 |
| Oxalic Acid | H2C2O4 | 5.6 x 10-2 |
| HC2O4- | 5.1 x 10-5 | |
| Phosphoric Acid | H3PO4 | 6.9 x 10-3 |
| H2PO4- | 6.2 x 10-8 | |
| HPO4-2 | 4.8 x 10-13 | |
| Phosphorous Acid | H2PHO3 | 1.6 x 10-2 |
| HPHO3- | 7 x 10-7 | |
| Propionic Acid | HC3H5O2 | 1.3 x 10-5 |
| Pyruvic Acid | HC3H3O3 | 1.4 x 10-4 |
| Sulfurous Acid | H2SO3 | 1.3 x 10-2 |
| HSO3- | 6.3 x 10-8 |
The base Kb's are as follows:
| Substance | Formula | Kb |
|---|---|---|
| Ammonia | NH3 | 1.8 x 10-5 |
| Aniline | C6H5NH2 | 4.2 x 10-10 |
| Dimethylamine | (CH3)3NH | 5.1 x 10-4 |
| Ethylamine | C2H5NH2 | 4.7 x 10-4 |
| Hydrazine | N2H4 | 1.7 x 10-6 |
| Hydroxylamine | NH2OH | 1.1 x 10-8 |
| Methylamine | CH3NH2 | 4.4 x 10-4 |
| Pyridine | C5H5N | 1.4 x 10-9 |
| Urea | NH2CONH2 | 1.5 x 10-14 |
Calculate the pH of a 0.100 M solution of HClO
| HClO | <--> | H+ | + | ClO- |
| .100 - x | x | x |
| Ka = | __x2__ |
| .100 - x |
The x in the denominator can be dropped because Ka/M is less than 10-3. If Ka/M is greater than 10-3 you have to use the quadratic formula to solve the equation.
Therefore:
| 3.5 x 10-8 = | __x2__ |
| .100 |
3.5 x 10-9 = x2
x = 5.9 x 10-5
[H+] = 5.9 x 10-5 M
[ClO-] = 5.9 x 10-5 M
[HClO] = .100 M - 5.9 x 10-5 ~= .100 M
pH = -log(5.9 x 10-5) = 4.2
Weak Base Example:
What is the concentration of OH- of a .20 molar solution of aniline?
| C6H5NH2 | <--> | C6H5NH3+ | + | OH- |
| .20 - x | x | x |
| Kb = | __x2__ |
| .20 - x |
The x in the denominator can be dropped because Kb/M is less than 10-3. If Kb/M is greater than 10-3 you have to use the quadratic formula to solve the equation.
Therefore:
| Kb = | __x2__ |
| .20 |
x2 = 8.4 x 10-11
x = 9.2 x 10-6
[OH-] = 9.2 x 10-6
![]()
![]()